FDA Approves First Oral Wegovy Pill for Weight Loss, Marking a New Era in Obesity Treatment
- bykrish rathore
- 24 December, 2025
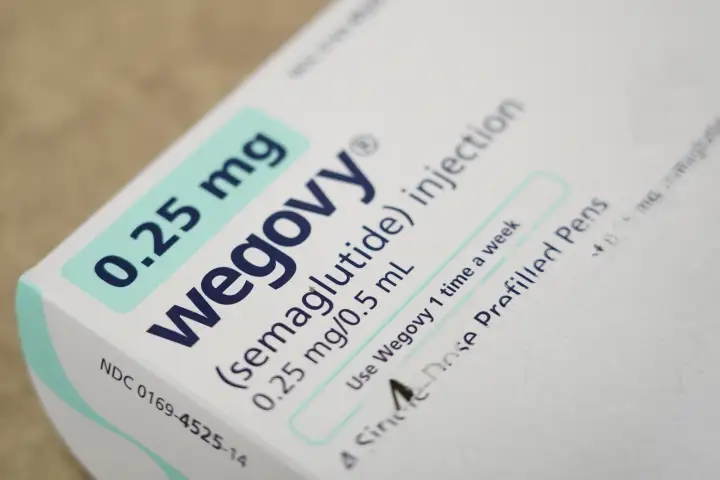
In a landmark decision for obesity treatment, the U.S. Food and Drug Administration (FDA) has approved the first oral version of the weight‑loss drug Wegovy, developed by pharmaceutical company Novo Nordisk. This once‑daily pill offers a new, non‑injectable option for adults living with obesity or those who are overweight with at least one weight‑related medical condition — such as high blood pressure or type 2 diabetes — when used alongside a reduced‑calorie diet and increased physical activity.
The newly approved Wegovy® pill contains 25 mg of semaglutide, the same active ingredient used in the injectable form that has been widely prescribed for weight management. Semaglutide belongs to a class of medications called GLP‑1 receptor agonists, which mimic a naturally occurring hormone that regulates appetite and food intake. By helping individuals feel fuller sooner and longer, the drug supports sustained weight loss over time.
Clinical trials played a key role in securing FDA approval. In the OASIS‑4 Phase 3 trial, adults taking the once‑daily Wegovy pill experienced significant weight loss compared with those taking a placebo. Trial participants saw an average reduction in body weight of around 16.6 % after 64 weeks when they remained ongoing with the treatment. Even when accounting for all participants regardless of treatment adherence, those on the pill lost approximately 14 % of their body weight versus about 2 % for the placebo group. PR
Importantly, the Wegovy pill is also approved to help reduce the risk of major adverse cardiovascular events — such as heart attacks and strokes — in adults with established heart disease and obesity or overweight. This dual benefit underlines the medication’s potential to improve both weight outcomes and overall cardiovascular health.
One of the biggest advantages of the oral formulation is convenience and patient preference. While injectable versions of GLP‑1 drugs — including Wegovy — have transformed obesity care, many patients have cited discomfort or hesitation around injections. The daily pill could improve adherence and broaden access for individuals who are reluctant to use injectable therapies.
The Wegovy pill is expected to hit the U.S. market in early 2026, with some pricing starting around $149 per month for patients paying out‑of‑pocket, although costs may vary with insurance coverage. Novo Nordisk has also engaged in programs and discount arrangements aimed at improving affordability for Medicare, Medicaid, and uninsured patients.
This approval marks a major milestone in the evolution of obesity treatment, expanding options beyond injections and signaling a significant shift in how chronic weight management can be approached. With obesity affecting millions of adults globally, the availability of an effective oral medication could have broad public health implications, offering a new tool to help individuals achieve lasting weight loss and better health outcomes.

Note: Content and images are for informational use only. For any concerns, contact us at info@rajasthaninews.com.
40 के बाद शर्ट से बा...
Related Post
Hot Categories
Recent News
Daily Newsletter
Get all the top stories from Blogs to keep track.


_1770194439.jpg)






